Periodic Table Concept Map
Learn concept map periodic table with free interactive flashcards.
Periodic table concept map. Atoms elements 2 development of the atom 3. Elements in the same group had the same valency and similar chemical properties. Periodic table concepts by hunter jelden 1. Choose from 265 different sets of concept map periodic table flashcards on quizlet.
This a chemistry resource aimed at ks4 and upper ks3 with 6 different concept maps for students to complete. Kaysons education basic concept of periodic table page 1 chapter day 1 periodic table 1. They discuss relationships between words thus exploring and revealing their own understanding. Based on the.
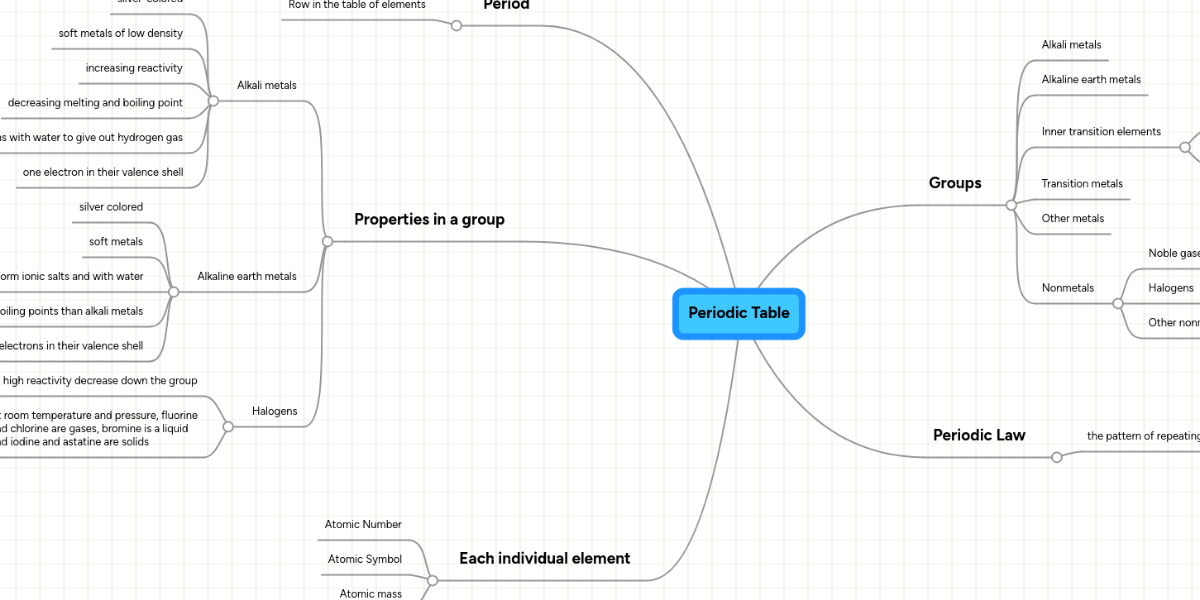
This trend in properties is known as periodic properties. The areas that students show the most difficulty with is providing words that connect the main concept words with one another and finding a way to connect atomic number and mass in a structured coherent way. 1 1 periodic table 1 2 periodicity of properties as per modern periodic. The two scientists who had the biggest impact were dmitri mendeleev and lothar meyer.
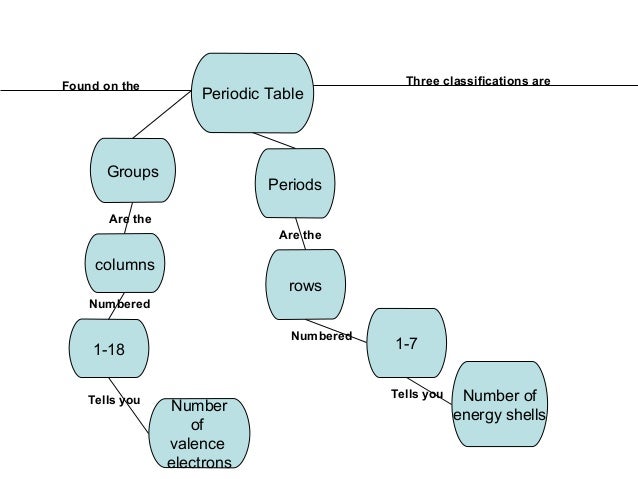
Just as a country s location on the globe gives you information about its climate an atom s position on the periodic table indicates the properties of its element. The modern periodic table is based on the law that the properties of an element are a periodic function of their atomic number. Groups 7 and 0 5. We observe a common trend in properties as we move across a period from left to right or down the group.
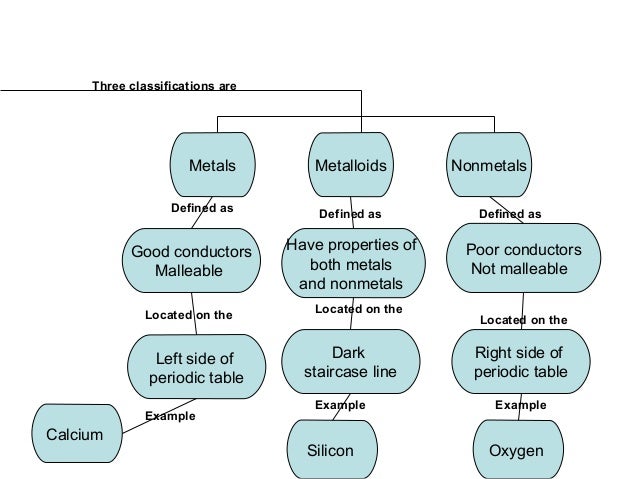
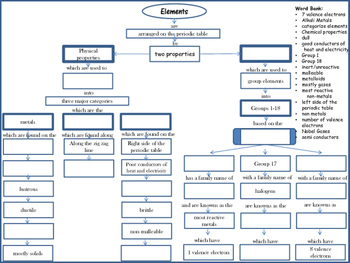
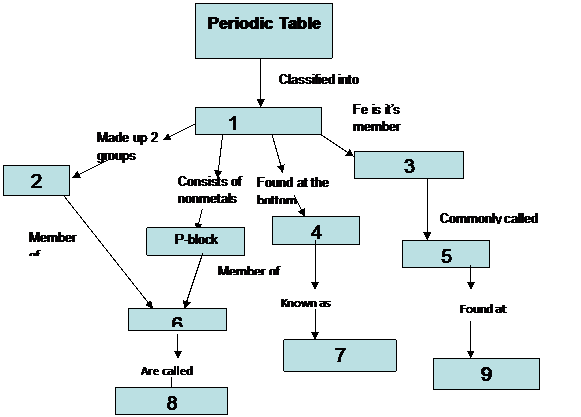
In this activity students use a concept map linking key words about the periodic table. Meyer used atomic weights to arrange 28 elements into 6 families that bore similar chemical and physical. Quickly memorize the terms phrases and much more. The important periodic properties are atomic size metallic character non metallic character ionization potential.
Elements were arranged in increasing order of atomic weights in horizontal rows called periods and vertical columns called groups. Elements which are similar with respect to their chemical properties are grouped together and have atomic weights of nearly the same value. Development of periodic table and 4. Mass size and electronic structure.
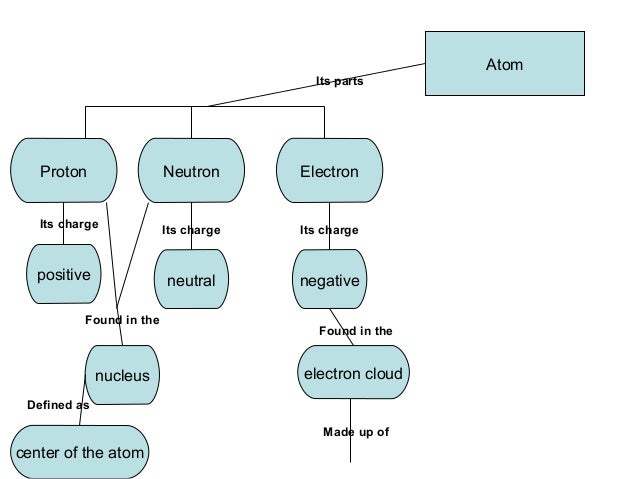
These properties are related to the electronic configuration of the elements. Mendeleev s original periodic table of elements first three periods shown above merits of mendeleev s periodic table. Mendeleev arranged the elements in his periodic table in order of increasing atomic mass 1 3. Since the goal of the lesson is mastery of the atom i show a version of the atom concept map that i created so students can revise their concept map cm for homework if they choose to do so.
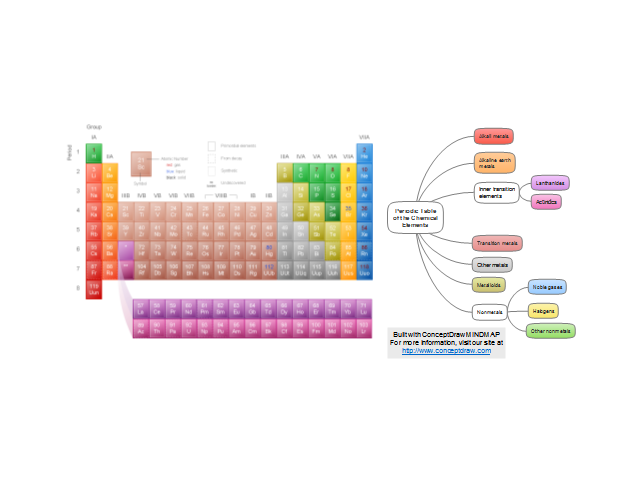
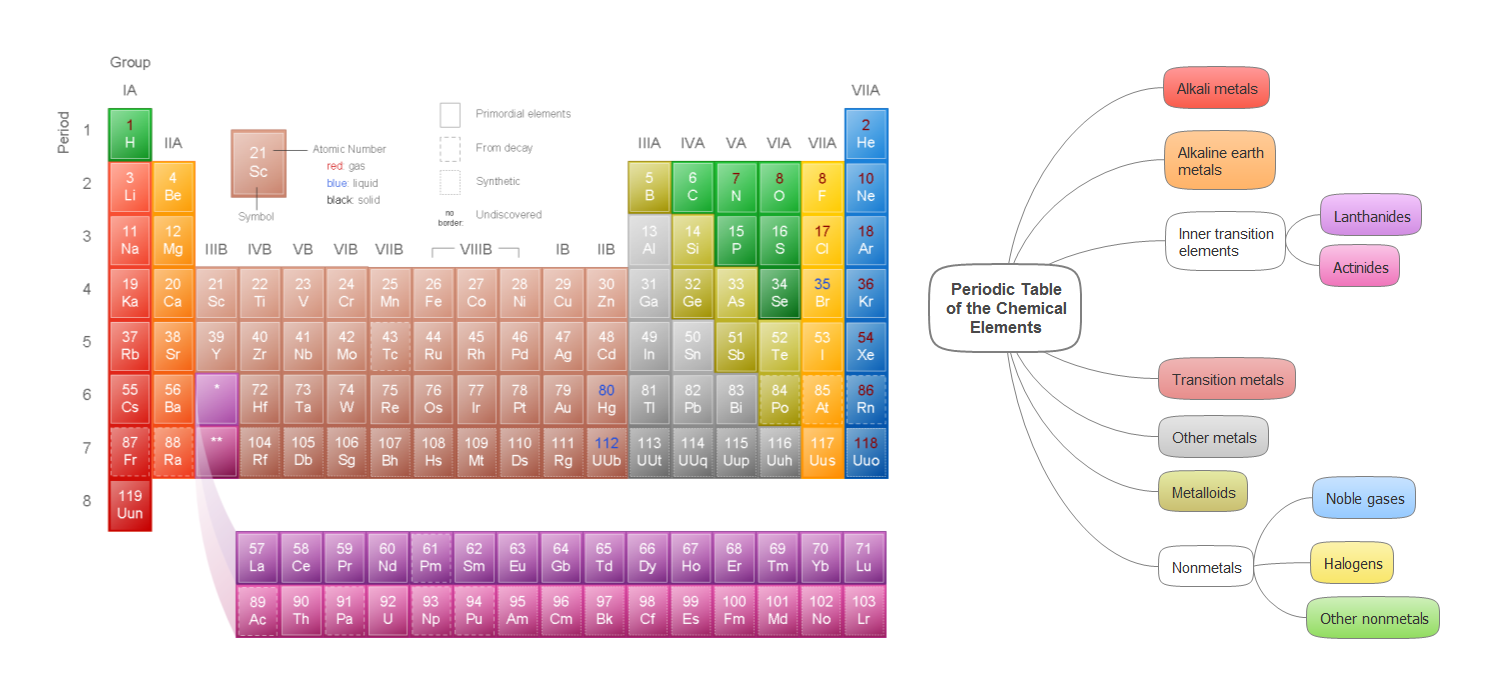
The periodic table is a kind of map of the elements.